
The Aedes genus of mosquito transmits many viruses, including Zika, dengue, chikungunya, West Nile and yellow fever.
© WS photography/Moment/Getty
Chikungunya, dengue and Zika viruses are transmitted by the same mosquito species — Aedes Aegypti and Aedes albopictus — and exhibit similar symptoms in infected patients. But, compared with the painful fever, headache and joint pain often associated with chikungunya, which means ‘to walk bent over’ in the Kimakonde language, and dengue, also known as ‘breakbone’ or ‘breakheart’ fever, Zika is like a mild flu, and often comes and goes unnoticed.
Before the Zika outbreak in northeast Brazil in May 2015, victims of mosquito bites might have preferred a diagnosis of Zika as its symptoms appeared milder than those of chikungunya or dengue. It wasn’t until doctors observed that women who had contracted the disease were delivering babies with severe birth defects that the medical profession realized that Zika was not as benign as first thought.
Like their international peers, researchers at A*STAR were intrigued by the emerging link between the virus and the outcome for unborn babies.
“We were surprised, concerned and a little disbelieving,” says Lisa Ng, a viral immunologist at the A*STAR Singapore Immunology Network. Ng is one of the many scientists at A*STAR working to improve understanding of the Zika virus and developing diagnostic and therapeutic tools to fight the disease. “Just like everyone else, I wondered how the virus could cause such an outcome — there are a lot of questions that need to be answered.”
Spreading fast

Researchers from multiple institutes at A*STAR are collaborating to develop diagnostic and therapeutic tools to fight the Zika virus. From left: Tan Heng Liang, Andre Choo, Lisa Ng, Masafumi Inoue and Sebastian Maurer-Stroh.
© 2016 A*STAR
The Zika virus has been around for a long time. First identified in rhesus macaques in the Zika forests of Uganda in 1947, it soon spread to a handful of humans, but was confined to Africa and parts of South East Asia. The first major Zika outbreak occurred on Yap Island, Micronesia, in 2007, infecting fewer than 100 people. In 2013, Zika re-emerged in French Polynesia, this time infecting an estimated 9,000 individuals. The international media and public health community largely overlooked these reports. It wasn’t until the virus’s next appearance in Brazil in 2015, with almost 1.5 million suspected cases recorded so far, that the world started to take notice.
By September 2015, doctors in Zika-affected areas were reporting an increase in the number of newborns with extremely small heads, a rare condition known as microcephaly. “Historically there have been cases of the rubella virus and certain drugs causing microcephaly. But to have a mosquito-borne virus causing it is something new,” explains Ng. “That is when everyone started freaking out — so many unknowns can be frightening.”
On 1 February 2016, the World Health Organization (WHO) declared the Zika-linked neurological disorders a public health emergency. And by May, scientists in the United States had established a causal link between the Zika virus, microcephaly and other severe brain defects. Over the past six months, almost 7,000 infants have been born with neurological disorders in Brazil, compared with fewer than 200 babies born with the defect between 2001 and 2014. A retrospective analysis of Zika-infected pregnant mothers in French Polynesia found similar cases of children with microcephaly. The virus has now spread to 60 countries, many for the first time. There are no treatments or vaccines against the Zika virus, nor for the chikungunya virus.
Researchers at A*STAR, along with other members of the scientific community, are now trying to answer the flood of questions raised by the Zika virus. How does the virus infect different cells in the body? How does the infection lead to microcephaly indeveloping fetuses? Why have these effects not been detected before? A*STAR researchers are collaborating with colleagues within the institute to develop diagnostic kits, including tests that can assess whether women of childbearing age have ever been infected with the disease.
“We have all been building expertise in our different fields over the last few years, and now it’s exciting to see everything come together in a way that addresses a problem relevant to the world,” says Andre Choo, a bioengineer at the A*STAR Bioprocessing Technology Institute (BTI), on the joint effort.
Early warning system

Masafumi Inoue at the A*STAR Experimental Therapeutics Centre and Sebastian Maurer-Stroh at the A*STAR Bioinformatics Institute have developed a quick and easy diagnostic test for dengue, chikungunya and Zika.
© 2016 A*STAR
Two days after the WHO announcement, Singapore’s Ministry of Health stepped up efforts to protect the island-nation against the Zika virus, including a program of clinical surveillance to correctly diagnose infected patients. The Environmental Health Institute, a public health lab at the National Environment Agency (NEA), teamed up with Masafumi Inoue at the A*STAR Experimental Therapeutics Centre, Sebastian Maurer-Stroh at the A*STAR Bioinformatics Institute, and clinicians at Tan Tock Seng Hospital, to develop a quick and easy diagnostic test.
The NEA had already been testing for Zika as part of their routine surveillance, but the team wanted to be able to monitor all three viruses, which presented similar symptoms in infected patients, at once — dengue, chikungunya and Zika. Singapore confirmed its first case of the Zika virus in May 2016, and sees an estimated 1,000 patients with chikungunya a year, but its heaviest public health burden comes from dengue, with an expected 30,000 cases in 2016.
Inoue, a molecular pathologist, had previously developed a similar five-combo test, or five-plex, for chikungunya and all four strains of dengue. Adding Zika to the test would be technically simple but impractical, since most hospitals do not have the equipment to process six separate tests at once. He would have to amalgamate the four dengue tests into one, creating a test that could detect all three infections.
The benefits of Inoue’s multiplex approach is its low cost and quick response. A diagnosis typically involves taking blood samples from a patient to look for signature sequences of genetic material made of ribonucleic acid (RNA) unique to a virus. For better detection, multiple copies of the RNA sequences are created through a well-known and highly sensitive process called polymerase chain reaction (PCR), using simple instruments found in most hospitals. “Other multiplex detection kits require expensive machines and special chips, which can cost up to 100 dollars a sample. Inoue’s test costs only a few dollars,” says Maurer-Stroh, bioinformatician. The entire test takes about two hours to provide results. “If we had to do each test separately, Tan Tock Seng Hospital would pile up with patients waiting up to eight hours before they could go home,” adds Inoue.
Inoue and Maurer-Stroh were familiar with high-intensity environments when a fast-moving infection becomes a public health emergency. Since the global swine flu epidemic in 2009, they have been working together on different aspects of influenza and virus diagnostics. The researchers were busy developing a test that could simultaneously detect pandemic and seasonal influenza, as well as the Middle East respiratory syndrome coronavirus (MERS-CoV), which had broken out in South Korea in May 2015, when reports began to emerge of a larger Zika outbreak in Brazil.
Sharing is caring
To develop their combined Zika, chikungunya and dengue test, Inoue began searching for a common region to detect all four variations of dengue. Meanwhile, Maurer-Stroh traced the historical evolution of the Zika virus genome. He discovered a crucial split between an African lineage, which spread west from Uganda to the Central African Republic, Nigeria and Senegal, and an Asian lineage, which spread east to Malaysia, Yap, French Polynesia and Brazil. Neurological complications, including cases of microcephaly, have only been observed in individuals infected with more recent versions of the Asian strain, suggesting that a genetic mutation could be responsible for the pathogenesis. But, molecular scientists have yet to prove this hypothesis. Inoue was careful to select a section of the genome found in both strains and not affected by mutations.
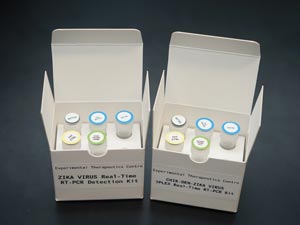
The combined diagnostic kit developed by Inoue and Maurer-Stroh will act as a critical early warning system for all three viruses — dengue, chikungunya and Zika — that exhibit similar symptoms in infected patients.
© 2016 Masafumi Inoue, A*STAR Experimental Therapeutics Centre
Within weeks, they had developed the combined test, but they did not have any live samples of Zika to confirm it could detect the virus in infected patients. It took another month to find and import a sample from a patient in Canada. In the meantime, Inoue synthesized viral RNA that could be used to at least prove that the kit worked. The kit (see image) is now being evaluated in hospitals, and the team plans to further improve the test to detect two other mosquito-borne viruses: yellow fever and West Nile. “We are very proud of our work and our contributions to controlling infectious disease outbreaks,” says Inoue — a contribution that extends beyond the diagnostics sphere.
After completing his molecular analysis of the different Zika strains, Maurer-Stroh did not just share it with Inoue. He communicated the results to colleagues and public health agencies across Singapore and fellow A*STAR researcher, Ng, shared it at a global consultation organized by the WHO in Geneva in March 2016. The slogan for the meeting read: ‘Stop Zika. Share data. Share samples.’
“Sharing data is important in emergency situations like Zika,” says Inoue. When, for example, Maurer-Stroh published an open access paper on an early computational analysis of the swine flu virus in 2009, it was accessed by individuals from 105 countries within the first year. “People have different strengths and technical capabilities. By sharing the data, you can make sure that the best people in their respective area of expertise can contribute,” says Maurer-Stroh. Of course, the risks of not being acknowledged for unpublished work are real, but he suggests that models of good governance do exist, such as the Global Initiative on Sharing All Influenza Data (GISAID). The publicly accessible platform sets clear guidelines on how to share, credit and access information for those who sign onto its terms of use.
“If I don’t realistically have a way of bringing the work further, but other people could, I make the work available independent of an emergency — for the sake of progressing our combined knowledge,” he says.
‘Zika virus was here’

Andre Choo and Tan Heng Liang at the A*STAR Bioprocessing Technology Institute are developing a longer-term test for identifying whether individuals have been infected with the Zika virus.
© 2016 A*STAR
Once deployed, Inoue and Maurer-Stroh’s diagnostic kit will act as a critical early warning system. But its window for detection is very narrow — only about a week after infection. Expectant mothers eager to find out if they have been infected with the Zika virus after this cutoff will have to wait for another test, also being developed at A*STAR.
When the Zika virus enters the body, the immune system typically mounts an offensive. These immune cells produce antibodies that can recognize unique features on the surface ofthe virus — similar to a crooked nose or a pointed ear on a human face — and mark it for elimination. Ng is working with Andre Choo and Tan Heng Liang at the BTI to generate antibodies that can spot the Zika virus, which if found in patient blood samples, would be like seeing ‘Zika virus was here’ spray-painted across an alley. “The antibody test would be as rapid as a pregnancy test,” says Choo, “and could be used to conduct diagnostics out in the field.”
Ng, Choo and Liang marshaled the resources to begin the urgent work of developing an antibody-based diagnostic test almost immediately. “We discussed the project at the end of the first week and by the second week we were into making the antibodies,” says Choo. “It is always exciting to conduct research in real-time — very different to ‘peacetime’ when we are all trying to answer a bigger problem at a comfortable pace,” says Ng. “The pressure is very high, which drives motivation.”
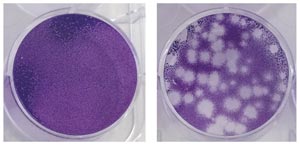
Mammalian cells infected with the Zika virus slowly begin to die (right).
© 2016 A*STAR Singapore Immunology Network
Their speed is the result of the group’s many years of combined experience. Ng’s lab specializes in the chikungunya virus, using animal models and patient cohorts to understand the disease’s mechanism of infection and immunity. She is trying to investigate similar pathways and pathologies of the Zika virus, for which her lab has already developed the relevant infection models in cells (see image) and organisms. Choo’s Antibody Discovery Platform is the go-to group for generating and harvesting antibodies that can eliminate stem cells and cancers.

Fluorescent images of immortal cells that can produce antibodies (red).
© 2016 A*STAR Bioprocessing Technology
To develop the Zika test, Ng infected mouse models with the Zika virus and collected their immune cells. Across the street, Choo and Tan fused these cells with immortal cells to ensure a continuous source of antibodies (see image). They then isolated about a hundred antibodies that could recognize and bind to the virus. A few will be further shortlisted for their ability to bind especially tightly to the Zika virus — important for diagnosis — and for their ability to block the virus from sneaking into and infecting host cells — potentially relevant for therapy. “Seeing these antibodies eventually go into applications that could benefit people is a very strong driving force for us, and the organization as awhole,” says Choo.
Unusually small heads
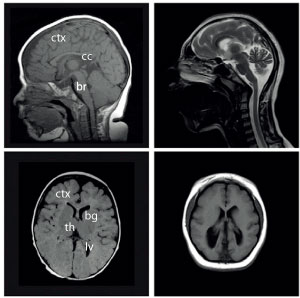
Magnetic resonance imaging scans of normal (left) and microcephalic (right) brains.
Reprinted from Neuron, Vol 84, Wen, F. et al, Katanin p80 regulates human cortical development by limiting centriole and cilia number, pp.1240–1257, Copyright (2014), with permission from Elsevier.
While many want to know what the Zika virus does once it enters the human body, even more people are keen to find out what it does to the brain cells of developing fetuses. Bruno Reversade, a molecular biologist at the A*STAR Institute of Medical Biology, is on the brink of answering some of these questions. His research is especially timely following a widely publicized paper published in The New England Journal of Medicine in May 2016 confirming that the Zika virus causes microcephaly.
Reversade’s research began in 2014, with individuals from three families in the Middle East who were found to have disproportionately small heads (see image). Given the rarity of the condition — before the recent Zika outbreak, only about one in every several thousand babies were born with microcephaly — Reversade and an international team of researchers thought these individuals might offer clues into the genetic origin of the deformities. The team eventually pinpointed the culprit gene in a protein responsible for slicing cellular scaffolding, katanin, named after the traditional Japanese samurai sword, katana. They have since identified several other genetic mutations associated with microcephaly.
Reversade is now applying his knowledge of microcephaly to understanding the pathogenesis of the Zika virus. He is collaborating with Ng to grow brain-like structures from developing neural cells, infect them with the Zika virus, and track the chain reaction. “It is like disease modeling in a dish,” explains Reversade. “This research highlights the power of rare and orphan genetic diseases as a means to understanding common threats.”
Reversade, like all of the scientists at A*STAR working on the Zika virus, is pleased about the many opportunities to conduct joint research. “Ng’s team brings a lot of tools and resources, while we have a much deeper knowledge of microcephaly — it makes a perfect team.”
“The beauty of A*STAR is that it can bring together scientists who are very well regarded in their respective fields to actually make a difference,” Reversade comments.
The A*STAR-affiliated researchers contributing to this research are from the Singapore Immunology Network, the Bioprocessing Technology Institute, the Experimental Therapeutics Centre, the Bioinformatics Institute and the Institute of Medical Biology.
About the Agency for Science, Technology and Research (A*STAR)
The Agency for Science, Technology and Research (A*STAR) is Singapore’s lead public sector agency that spearheads economic oriented research to advance scientific discovery and develop innovative technology. Through open innovation, we collaborate with our partners in both the public and private sectors to benefit society.
As a Science and Technology Organisation, A*STAR bridges the gap between academia and industry. Our research creates economic growth and jobs for Singapore, and enhances lives by contributing to societal benefits such as improving outcomes in healthcare, urban living, and sustainability.
We play a key role in nurturing and developing a diversity of talent and leaders in our Agency and Research Institutes, the wider research community and industry. A*STAR oversees 18 biomedical sciences and physical sciences and engineering research entities primarily located in Biopolis and Fusionopolis.




